opioid withdrawal
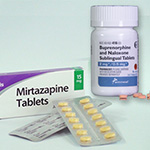
Could Mirtazapine Be Used to Ease Opioid Withdrawal?
Mirtazapine, an atypical antidepressant used to treat major depressive disorder, may also relieve several symptoms ...
SEPTEMBER 28, 2023
HHS Standardizes Definition of Opioid Withdrawal in Infants
The HHS has developed a standard clinical definition for neonatal opioid withdrawal in infants.
FEBRUARY 25, 2022
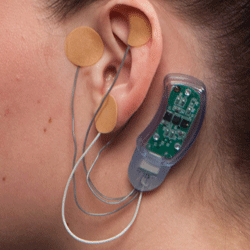
Wearable Neurostimulation Device Shown to Reduce Opioid Withdrawal Symptoms
New research has demonstrated the viability of transcutaneous transauricular neurostimulation to reduce the ...
MAY 21, 2021
Bridging the Gap to OUD Recovery: Study Looks at Buprenorphine Initiated in Emergency Department
Can emergency departments use buprenorphine to help bridge opioid-dependent patients on the road to recovery from ...
OCTOBER 14, 2020

Hyperbaric Oxygen Therapy Could Benefit Opioid Withdrawal
After only one day of hyperbaric oxygen therapy, opioid withdrawal symptoms were reduced by twice as much, on ...
MAY 4, 2020

HHS Announces Guide for Appropriate Tapering or Discontinuation of Long-Term Opioid Use
The Department of Health and Human Services has published a new guide designed to help clinicians reduce or ...
NOVEMBER 5, 2019
Alpha-Adrenergic Agonist Mitigates Abrupt Opioid Discontinuation–Related Withdrawal
For clinicians on the front line of the opioid crisis, there may be a new weapon against the significant symptom ...
AUGUST 29, 2019

Improved Treatment Compliance, Reduced Opioid Exposure Among Outcomes Seen With NP Service Line
A pediatric nurse practitioner service line may boost quality improvement metrics in evidence-based practice ...
JUNE 27, 2018

First Nonopioid Treatment for Withdrawal Approved by FDA
The FDA approved lofexidine hydrochloride, a first-of-its-kind nonopioid for treatment of opioid withdrawal.
MAY 18, 2018

FDA Committee Recommends Approval of Lofexidine for Opioid Withdrawal
In a nearly unanimous vote, an FDA committee recently recommended approval of lofexidine (Lucemyra, US WorldMeds), ...
APRIL 4, 2018

FDA, FTC Crackdown on Marketing Unapproved Opioid Addiction, Withdrawal Products
The FDA and FTC sent joint warning letters to the marketers and distributors of 12 opioid cessation products for ...
JANUARY 25, 2018

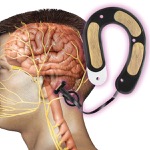
FDA Grants New Indication to NSS-2 Bridge to Reduce Opioid Withdrawal Symptoms
The NSS-2 Bridge emits electrical pulses to stimulate branches of certain cranial nerves for use in helping to ...
NOVEMBER 21, 2017