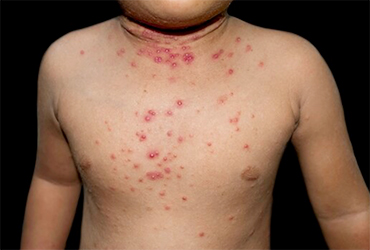
The FDA approved cantharidin (Ycanth/Verrica Pharmaceuticals), the first approved treatment for children and adults with molluscum contagiosum, a painful viral skin infection.
Involving primarily children, molluscum contagiosum affects approximately 6 million people in the United States. The highly contagious disease is transmitted through skin-to-skin contact and shared objects. The FDA issued a warning for the use of off-label molluscum treatments and self-diagnosis in June.
Cantharidin is for topical use only and targets the burdensome lesions associated with molluscum contagiosum. Applied with a single-use applicator, cantharidin induces blistering of the lesions, resulting in their removal from the patient’s skin. The treatment may be applied by a healthcare provider every three weeks as needed.
The FDA based its approval on two phase 3 trials in which cantharidin was shown to be safe and effective for patients as young as 24 months old.
The identical randomized, double-blind, multicenter clinical trials (CAMP-1 and CAMP-2) evaluated the safety and efficacy of cantharidin compared with placebo in patients 24 months old and older diagnosed with molluscum.
The primary end point of complete clearance of all treatable molluscum lesions was met by a clinically and statistically significant number of patients treated with cantharidin in both trials. In CAMP-1, nearly 50% of patients in the treatment cohort achieved complete clearance of molluscum lesions, compared with 18% in the vehicle group (P<0.0001). CAMP-2 saw similar results: 54% of treated patients achieved complete clearance of molluscum lesions, compared with 13% of the vehicle group (P<0.0001).
Additional post hoc analyses of the CAMP trials confirmed complete clearance of all lesions was statistically significantly higher in the treatment group than placebo across all body regions, including areas considered most sensitive.
During clinical trials, local skin reactions, including vesiculation, pruritus, pain, discoloration and erythema, were observed at the application site in 97% of patients treated with cantharidin. There were no serious adverse events reported in either trial, and adverse reactions were categorized as mostly mild to moderate. There was a 2.3% discontinuation rate among patients treated with cantharidin and 0.5% in the placebo group. See the package insert for more information for prescribing.
“Molluscum, which primarily affects children, is highly contagious and is commonly transmitted in households, schools, swimming pools and other extra-curricular settings,” said Ted White, the president and CEO of Verrica.
“Since molluscum spreads through skin-to-skin contact and the sharing of contaminated objects with its viral lesions, a topical treatment with precise administration is essential toward preventing further transmission,” Mr. White said.
The company plans to make the treatment available by September 2023.
—PMN Staff
From company press materials.
